Indication(s) And Safety Information
CARBAGLU® (carglumic acid) tablets for oral suspension 200 mg
INDICATIONS AND USAGE
CARBAGLU® (carglumic acid) is a carbamoyl phosphate synthetase 1 (CPS 1) activator indicated in adult and pediatric patients as:
Acute and Chronic Hyperammonemia due to N-acetylglutamate Synthase (NAGS) Deficiency:
- Adjunctive therapy to standard of care for the treatment of acute hyperammonemia due to N-acetylglutamate synthase (NAGS) deficiency.
- Maintenance therapy for the treatment of chronic hyperammonemia due to N-acetylglutamate synthase (NAGS) deficiency.
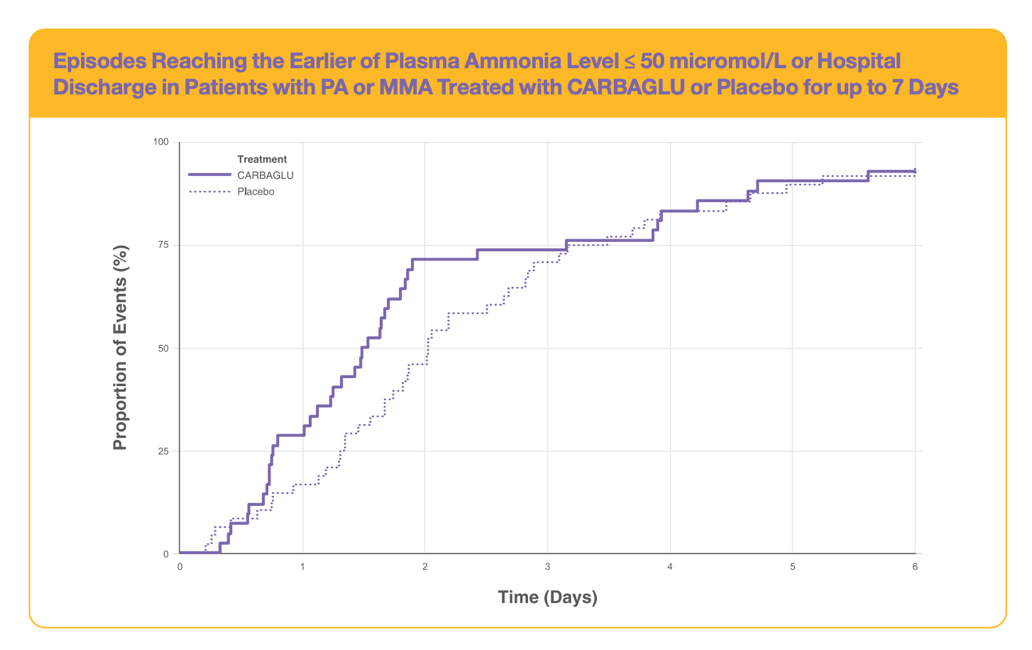
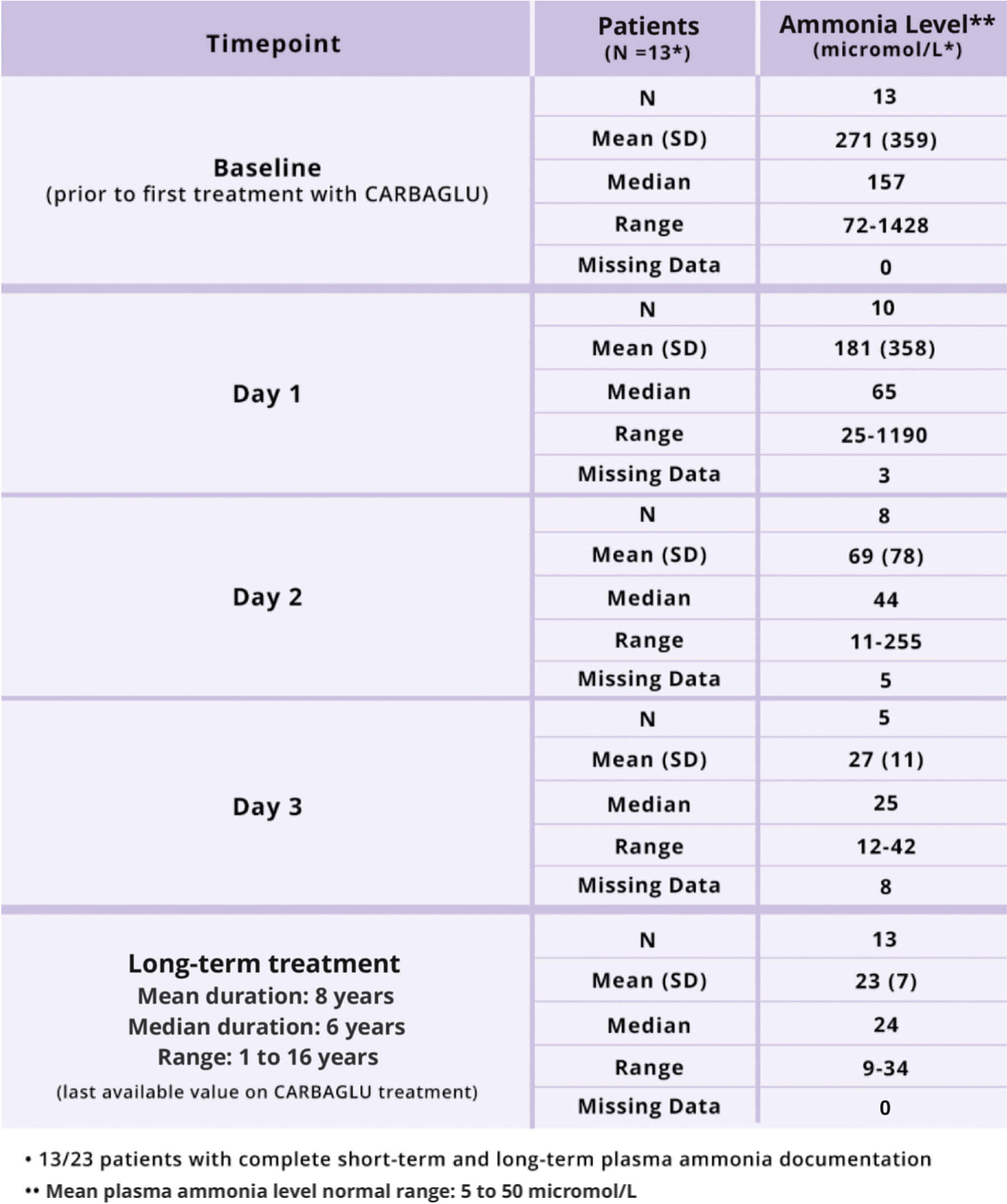
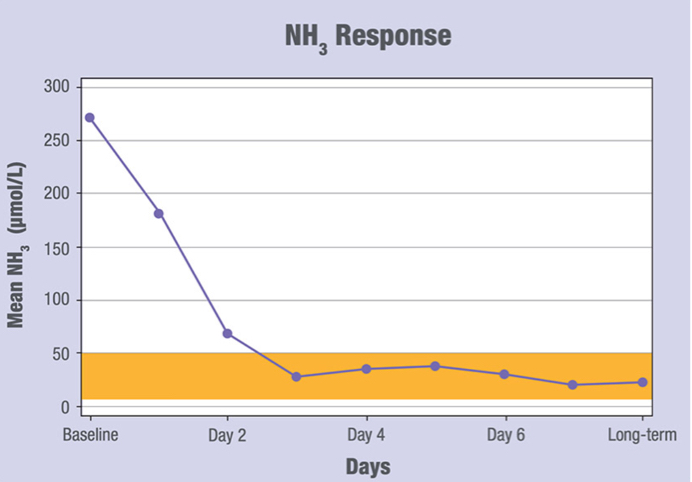
Acute Hyperammonemia due to Propionic Acidemia (PA) or Methylmalonic Acidemia (MMA)
- Adjunctive therapy to standard of care for the treatment of acute hyperammonemia due to propionic acidemia (PA) or methylmalonic acidemia (MMA).
Important Safety Information
Contraindications: None.
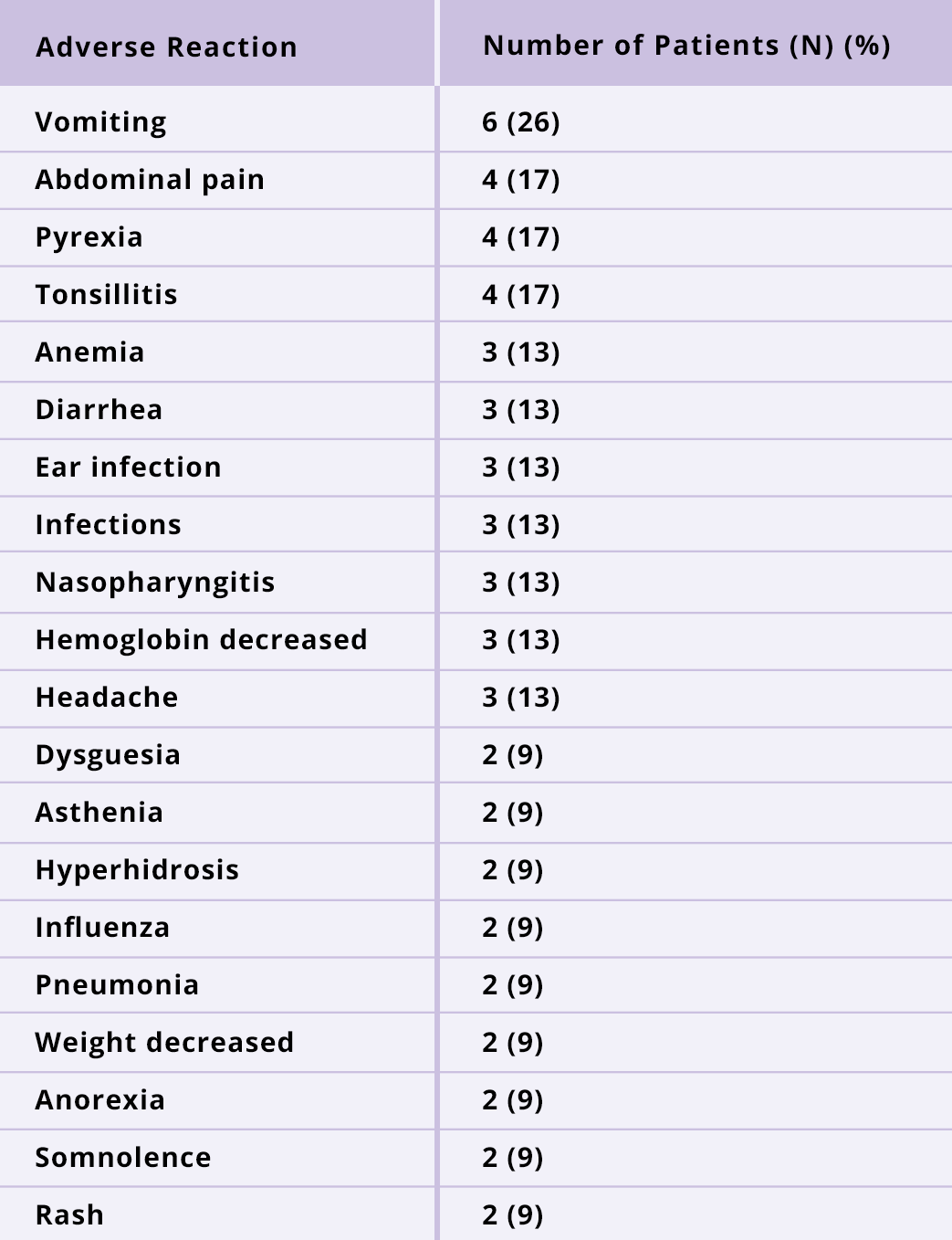
NAGS deficiency: The most common adverse reactions (≥13%) are vomiting, abdominal pain, pyrexia, tonsillitis, anemia, diarrhea, ear infection, infections, nasopharyngitis, hemoglobin decreased, and headache.
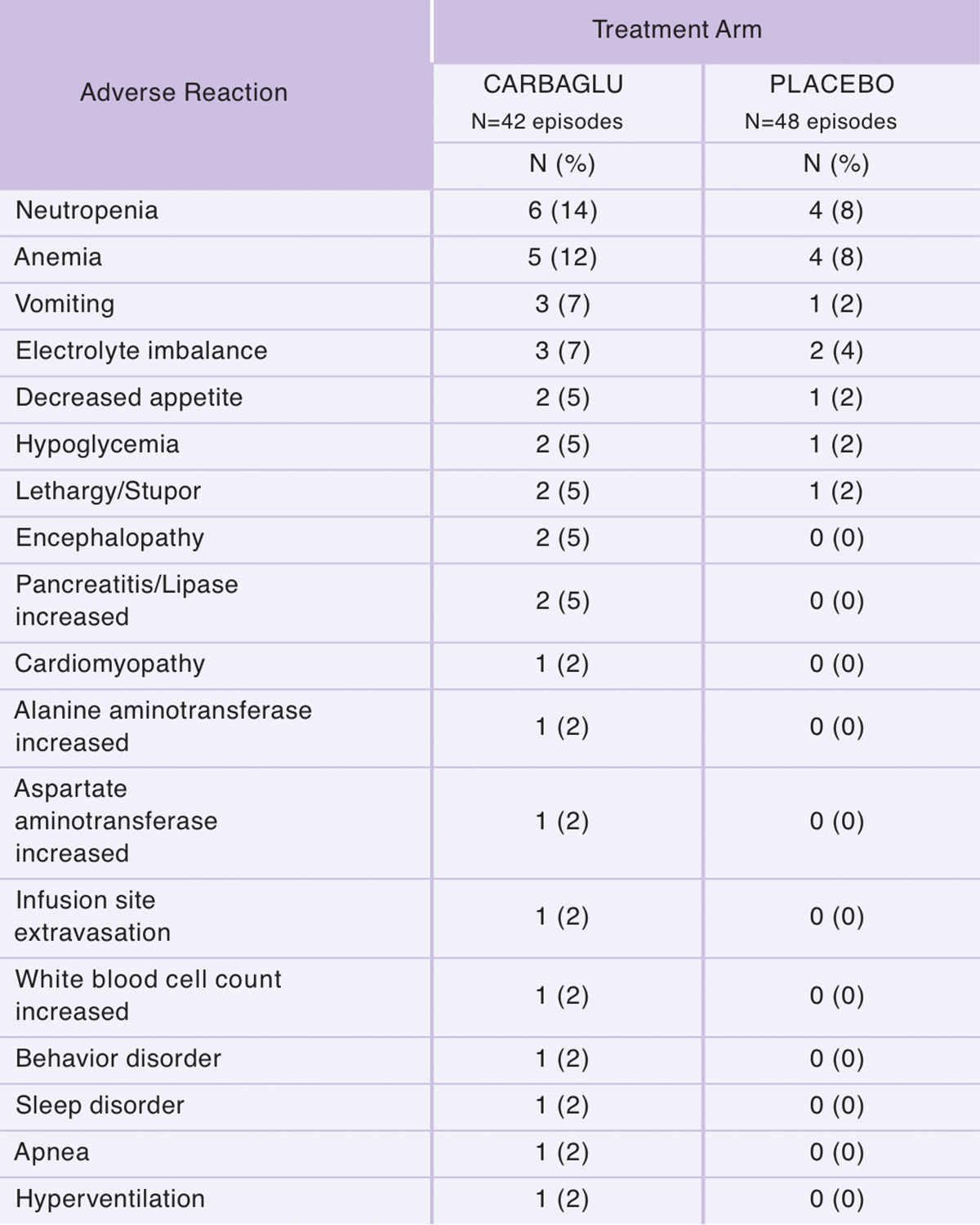
PA and MMA: The most common adverse reactions (≥5%) are neutropenia, anemia, vomiting, electrolyte imbalance, decreased appetite, hypoglycemia, lethargy/stupor, encephalopathy and pancreatitis/lipase increased.
To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use in Special Population(s): If CARBAGLU is administered during pregnancy to women with NAGS deficiency, health care providers should report CARBAGLU exposure to the pregnancy pharmacovigilance program by calling 1-888-575-8344.
Dosing and Administration: The recommended dosage of CARBAGLU in patients with moderate or severe renal impairment must be adjusted based upon the estimated glomerular filtration rate (eGFR). See Prescribing Information for details.
CARBAGLU® (carglumic acid) is available as 200 mg tablets for oral suspension.
For more information, please see full Prescribing Information at www.carbaglu.com/PI